Stoichiometry Calculations
Ever wondered how to calculate the exact amount of product in a chemical reaction? Stoichiometry is your answer! Let's break down some examples:
When working with chemical equations like C₁₈H₁₀N₂O₄ + H₂O₂ → Products, you need to use mole ratios from the balanced equation. For melanin oxidation, if 20g of a 9% H₂O₂ solution is used, you'd calculate the mass of H₂O₂ first (1.8g), then convert to moles and apply the mole ratio.
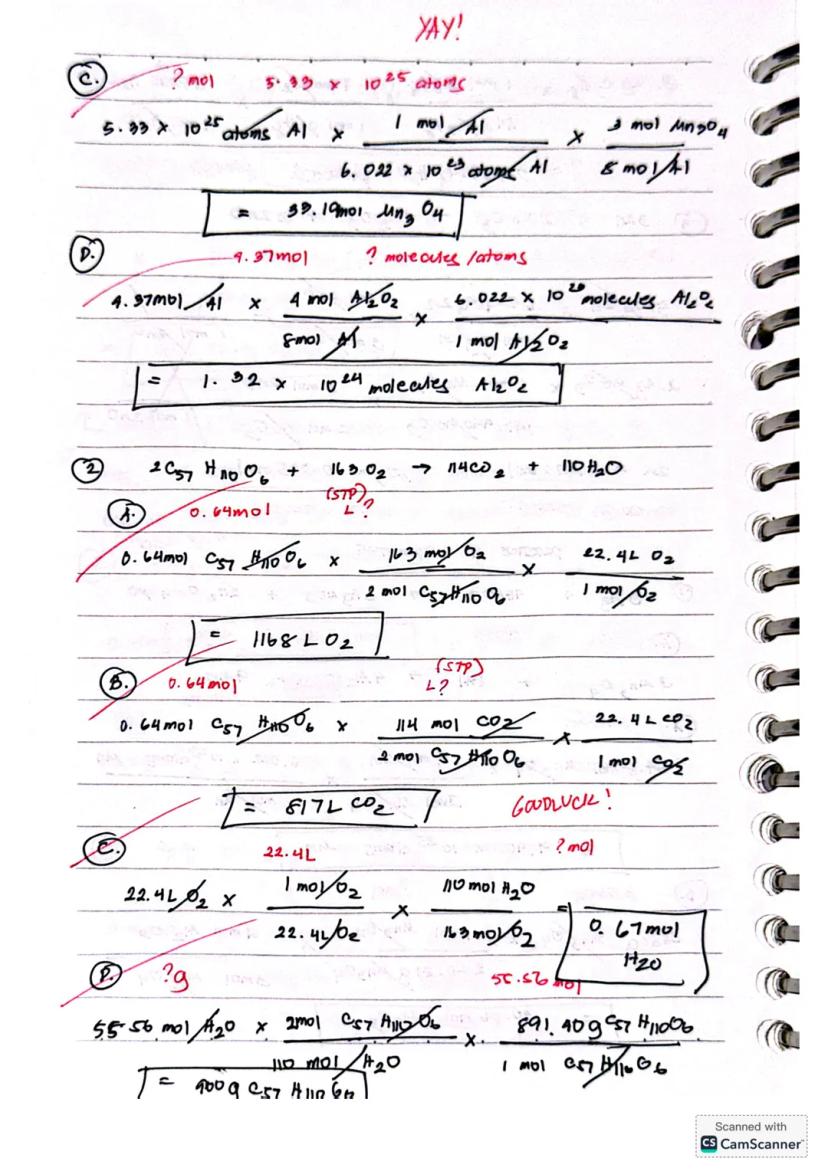
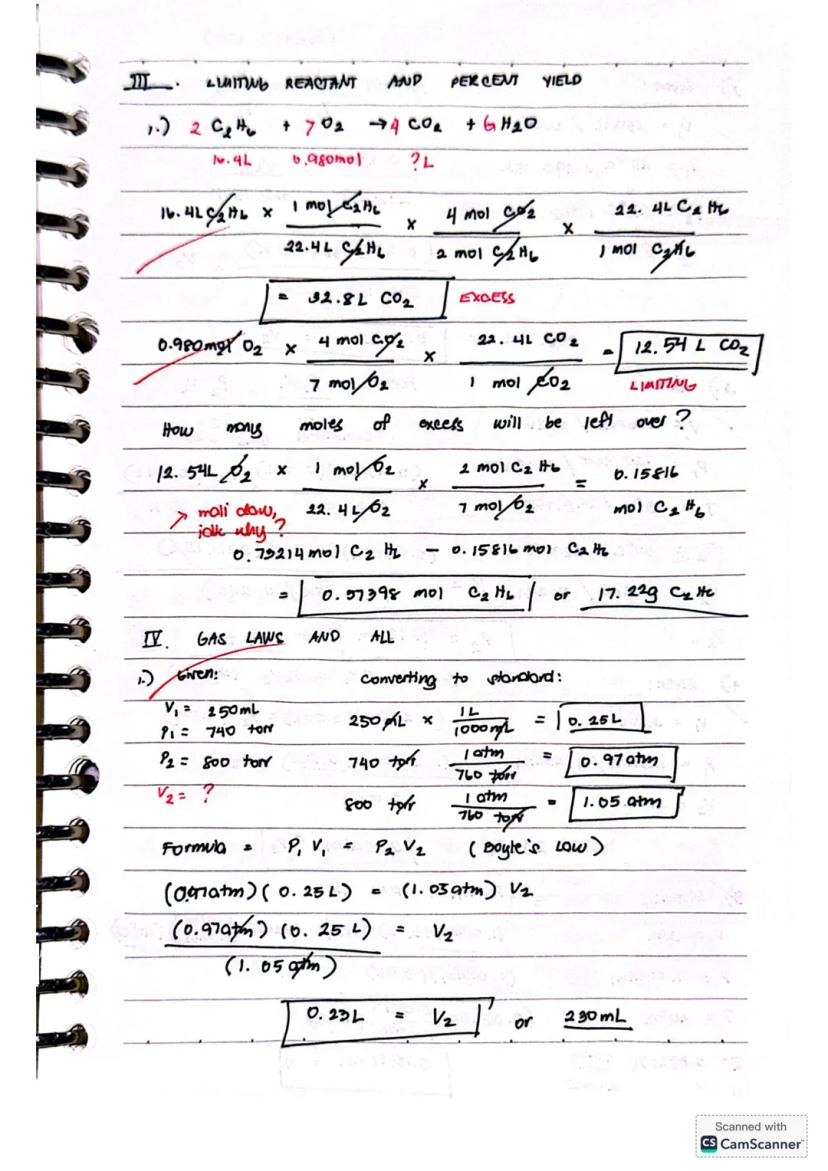
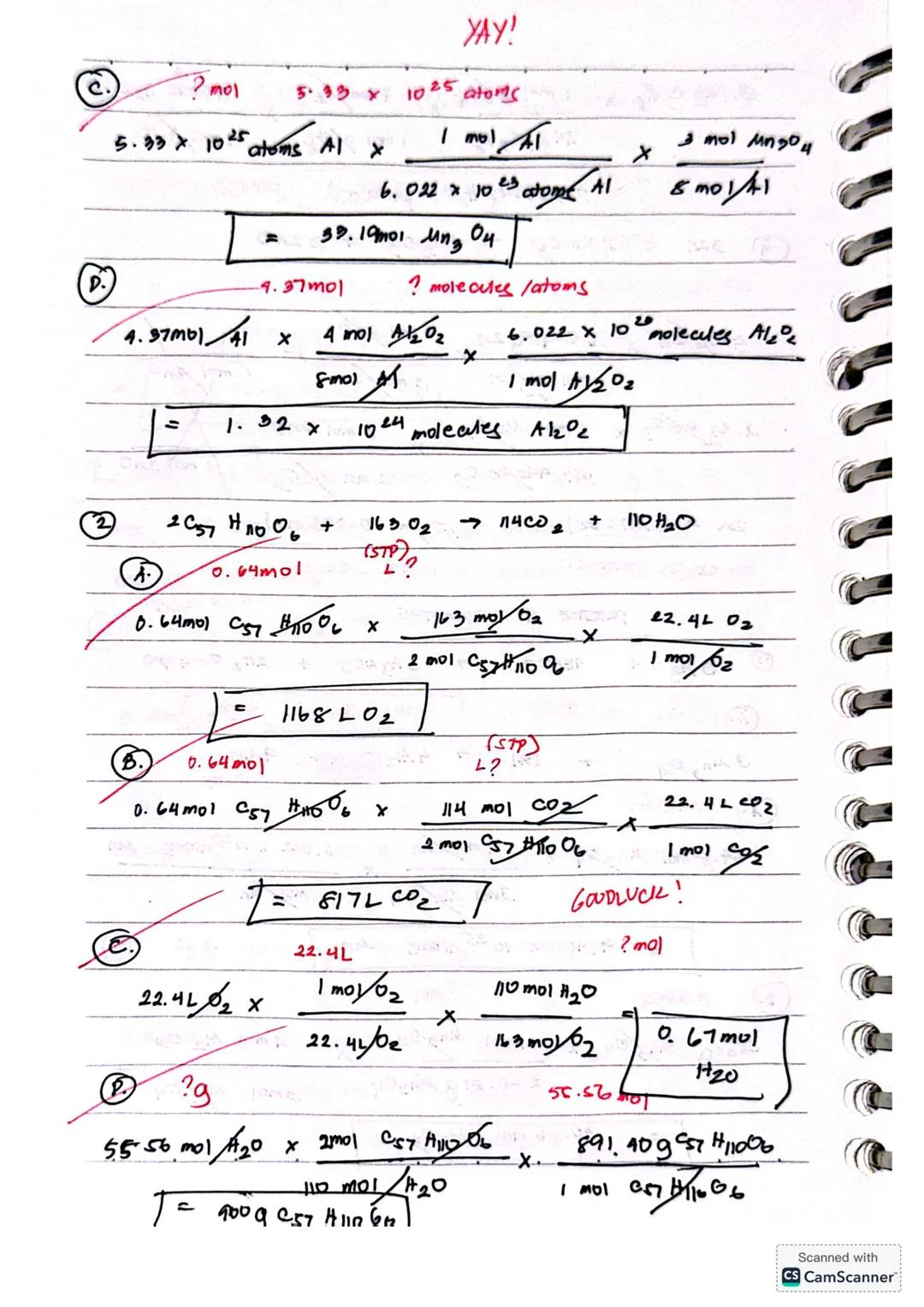
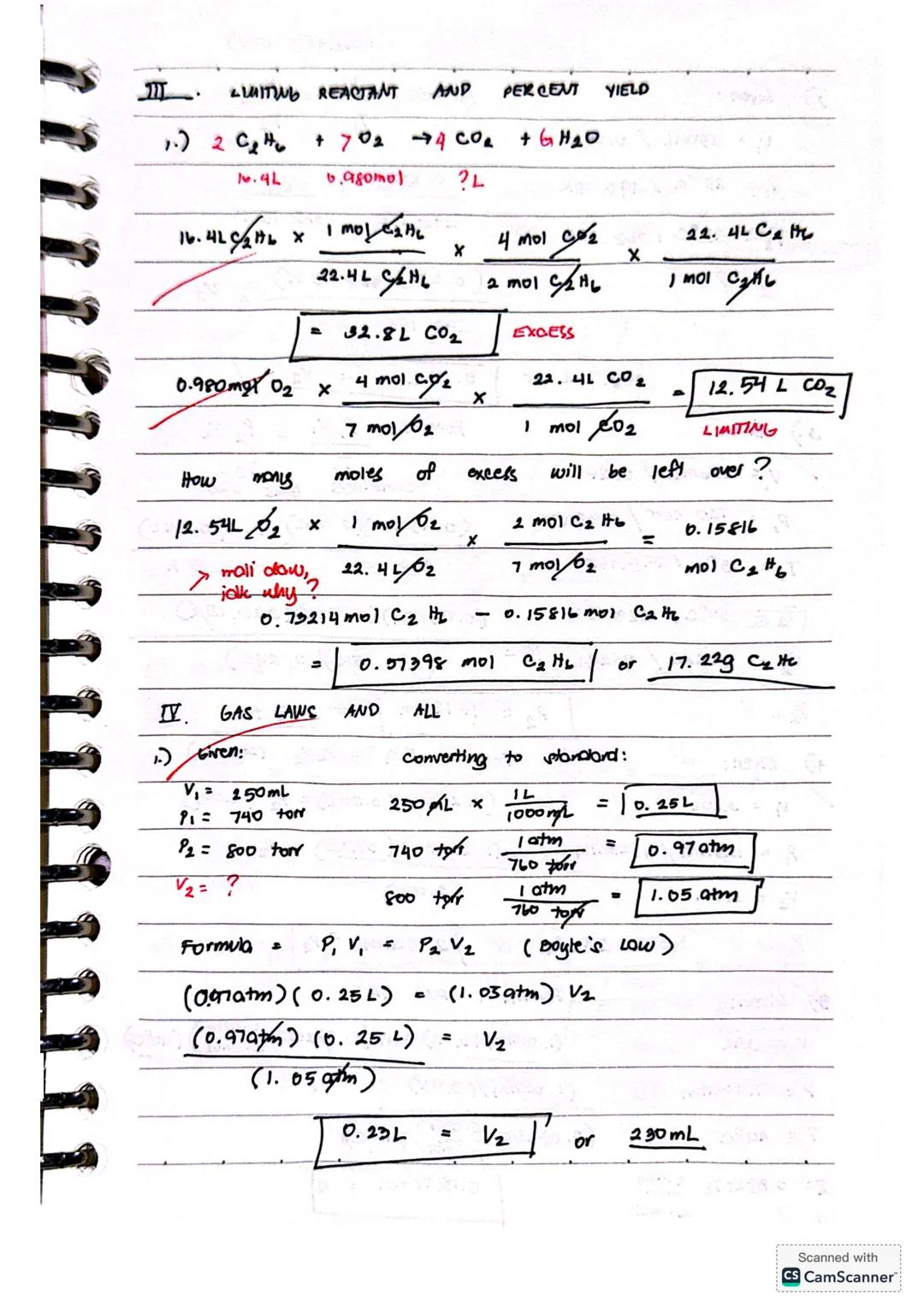
For reactions like 2BF₃ + 3H₂ → 2B + 6HF, identifying the limiting reactant is crucial. Compare the theoretical yields from each reactant - the one producing less product is your limiting reactant. For example, 0.10 mol BF₃ would produce 0.10 mol B, while 0.25 mol H₂ would yield 0.17 mol B, making BF₃ the limiting reactant.
💡 Quick Tip: Always convert to moles first, then apply mole ratios from the balanced equation, and finally convert to the requested unit (mass, volume, etc.).
When calculating product yield for 3C₉H₈ + 5O₂ → 3C₉O₂ + 4H₂O, the same process applies. If you have 3.4g O₂, you'd convert to moles, apply the mole ratio 4molH2O/5molO2, and then calculate the mass of water produced.