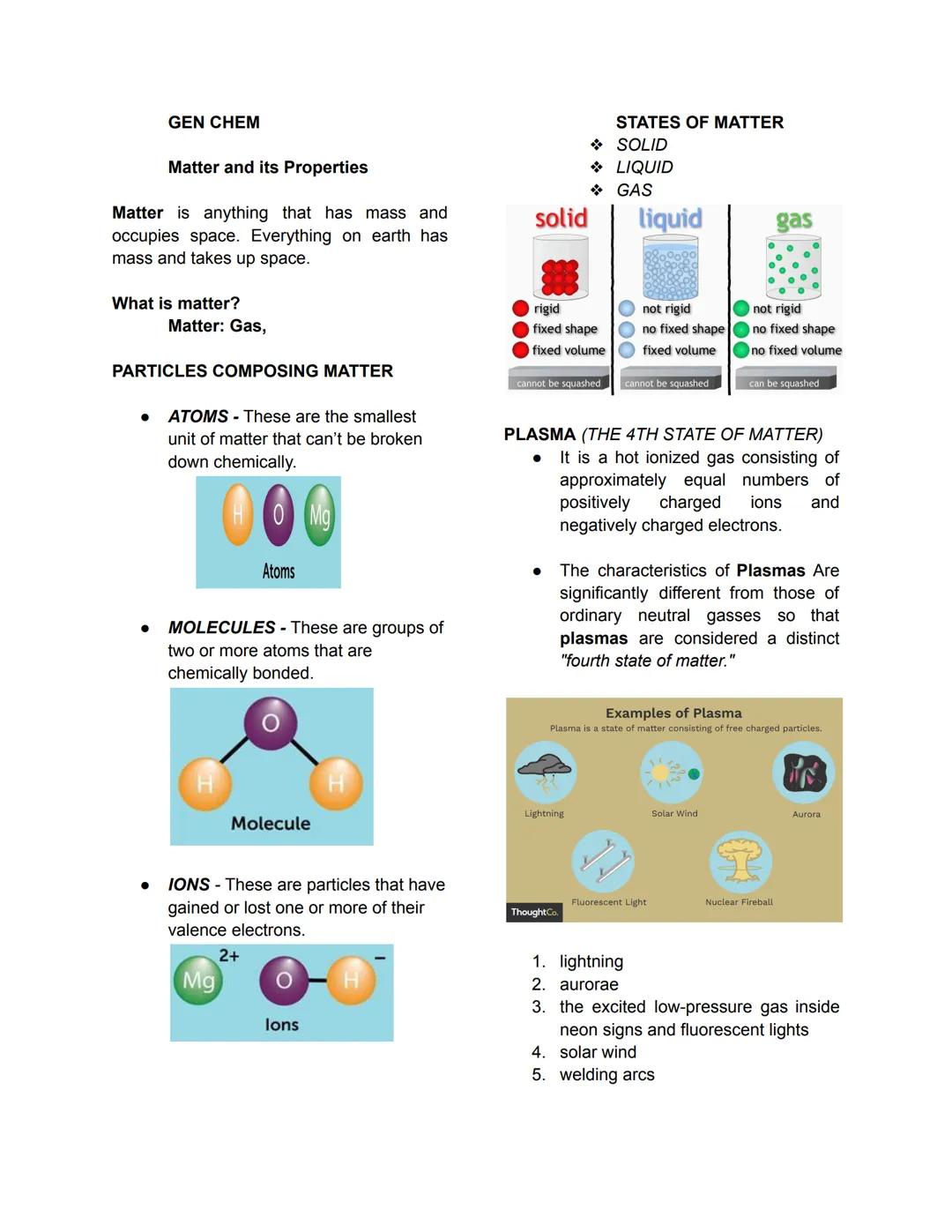
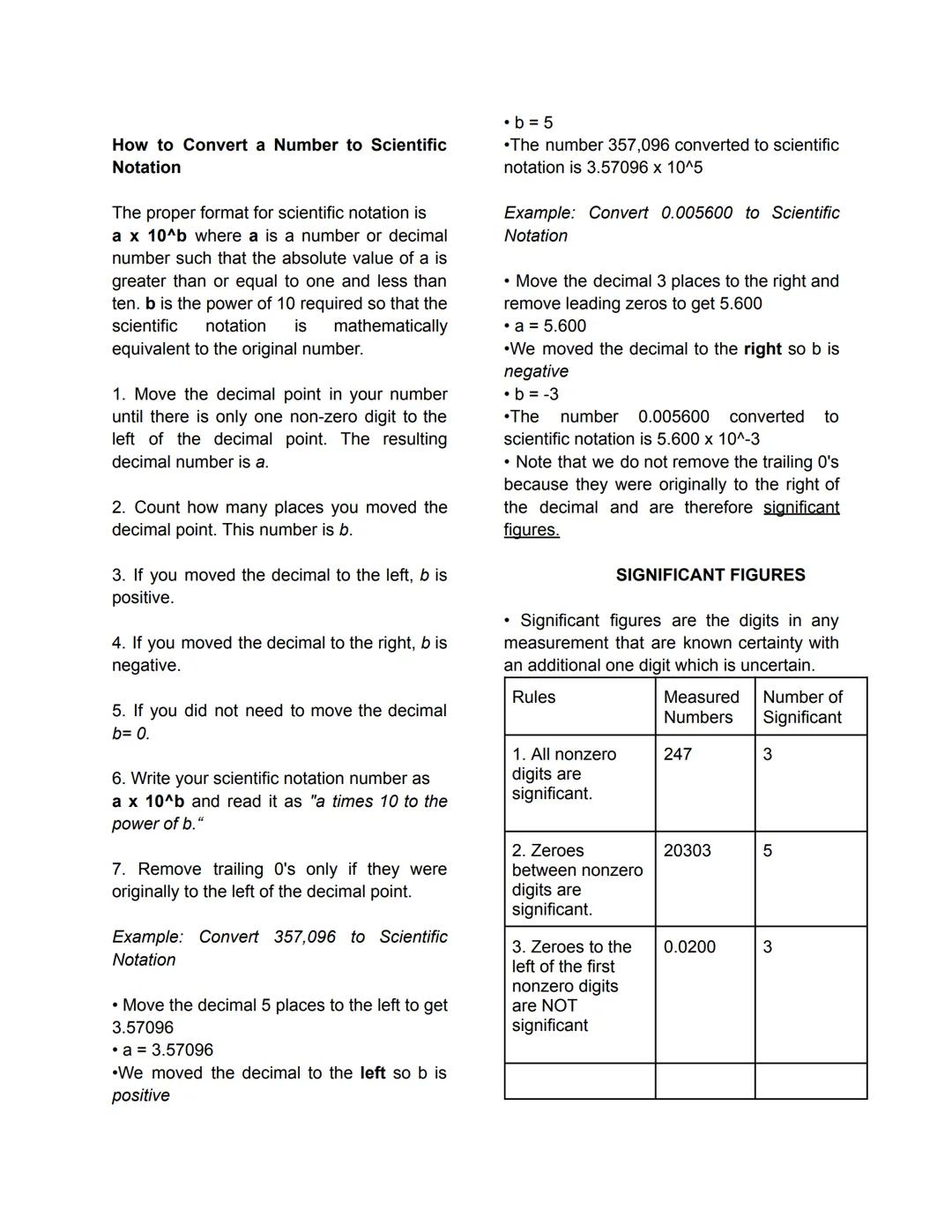
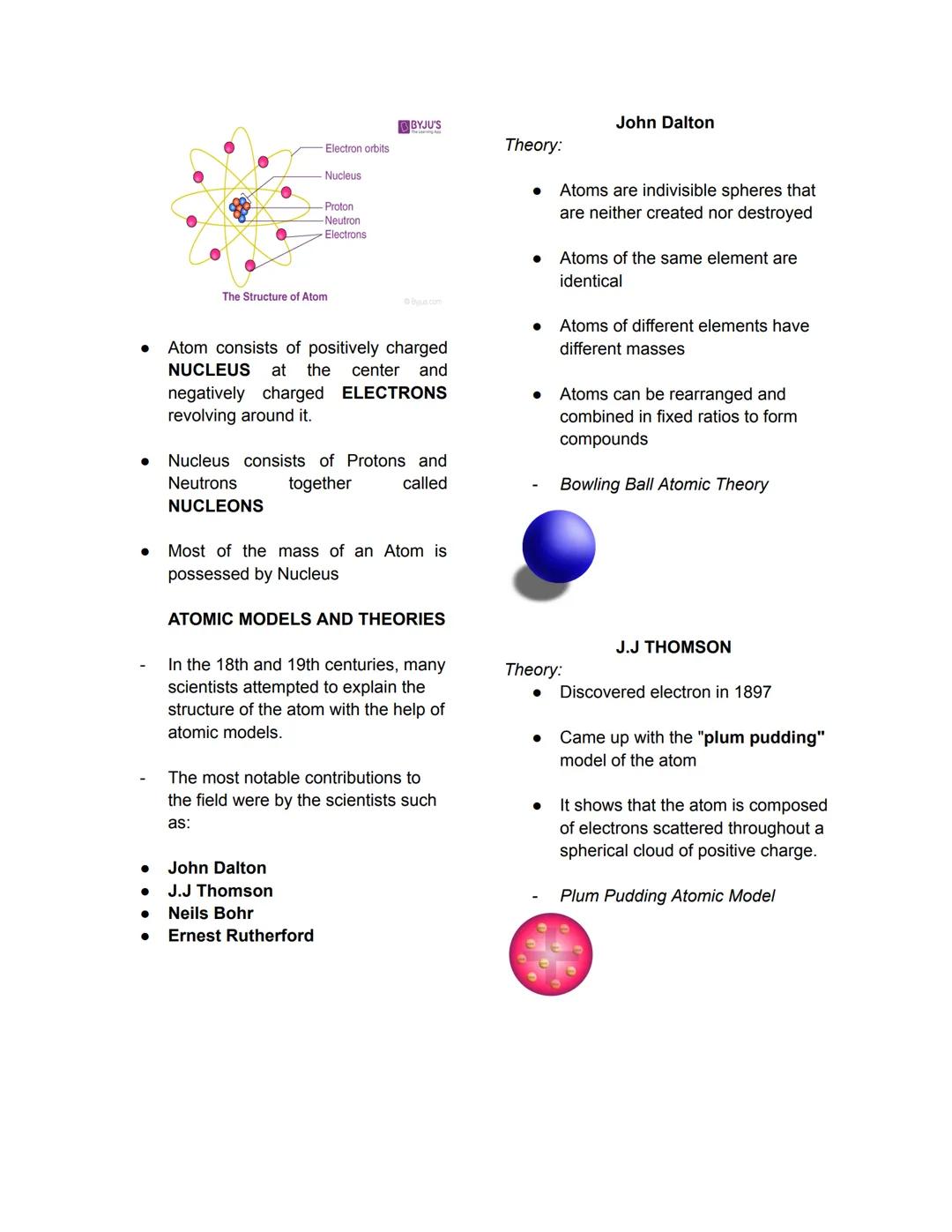
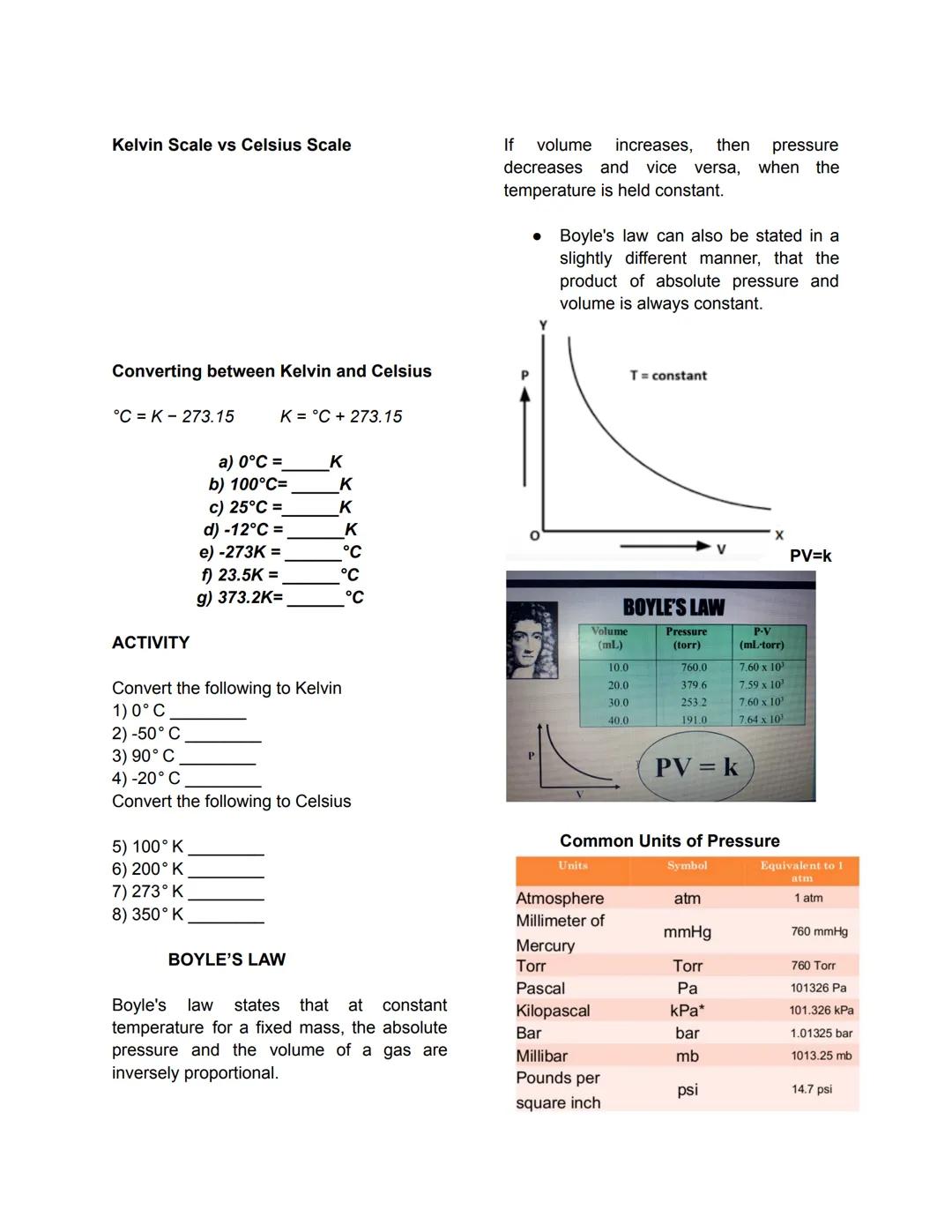
Pure Substances vs. Mixtures
Not all matter is created equal - some things are pure while others are mixed up! Pure substances have consistent composition throughout, like distilled water or table salt. These include elements (like oxygen) and compounds (like sugar).
Mixtures combine different substances that keep their individual properties. Homogeneous mixtures look uniform throughout (like soft drinks or milk), while heterogeneous mixtures have visible different parts (like oil and water or trail mix).
Here's a quick test: Can you see different components? If yes, it's heterogeneous. If it looks the same throughout but contains multiple substances, it's homogeneous.
Separating mixtures uses different techniques based on physical properties. Filtration catches solid particles, evaporation leaves behind dissolved solids, distillation separates liquids with different boiling points, and magnetic separation pulls out metals.
Real-world connection: When you make coffee, you're using filtration to separate liquid coffee from solid grounds!